|
|
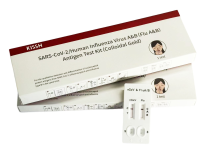
The novel coronavirus antigen detection reagent (colloidal gold method) developed and produced by company at present has obtained the European CE self-test certificate This new milestone signifies that its product quality is recognized by the European Union, and it allows the self-tested antigenic agent to be sold in the 27 Member States of the European Union and in the countries that recognize the CE certificate, broadening the way in which it will continue to contribute to the global response. Since the outbreak of COVID-19 in 2020, the world has faced unprecedented challenges in combating the disease. In order to relieve the pressure on the healthcare system, many countries have added antigen testing as a supplement to the original PCR test method. Therefore, in the international scope, there is no need for testing equipment, and the demand for antigen testing products with easy operation and fast results has increased significantly. Since its establishment, the company has been engaged in the development and manufacturing of IVD products and pathology products. Its research and development team keeps abreast of market needs and keeps a real-time eye on epidemic development. Its developed reagents detect not only Alpha, Beta, Gamma, Delta, and other mutant strains, It can also effectively detect the Omicron and its variant series that have recently swept the world.
Its CoV-2 antigen test kit (colloidal gold method) is a COV-2 antigen assay used for individual or home self-testing. It provides a rapid detection of COV-2 infection in a subject within 15 minutes.
The kit has been well received by agents and consumers since its launch, and its excellent performance in sensitivity and specificity has earned company a favorable reputation in overseas markets. The reagent not only successfully entered the EU HSC Common List, but also successively approved the EUA emergency channel access qualification of the UK MHRA, Germany BfArM, France ANSM, Thailand FDA, Malaysia MDA, Indonesia MOH and other countries and regions. The European self-testing CE certificate approved at this time is subject to a rigorous review by a third-party testing organization accredited by the European Union and a product clinical test evaluation, which further demonstrates the superiority and stability of the product's performance. Its company philosophy is "dedicated to human health", and it continues to conduct a company operation at a high standard, achieving its corporate goal of "making human health simpler and more intelligent". |