|
|
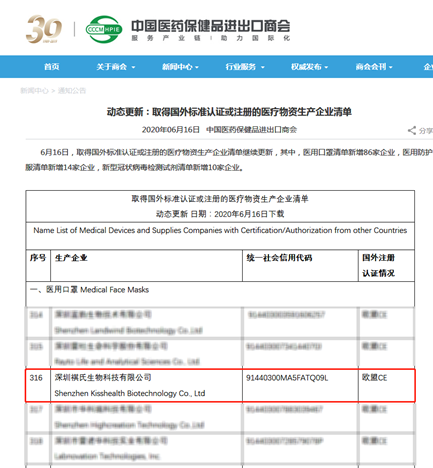
A few days ago, its self-developed CoV antibody detection reagent is on the "white list" of the China Chamber of Commerce for Import and Export of Pharmaceutical and Health Products, and it is also licensed in a number of countries. The serum total antibody detection method is a methodology recommended by the WHO and is suitable for rapid automated detection of large populations. At present, the company is committed to continuing to support the international community in fighting the epidemic. At this special time when the global epidemic continues to spread, in order to more effectively support the international community's joint response to the global public health crisis, it is now necessary to further strengthen the quality supervision of epidemic prevention materials and standardize the export order. On April 25, 2020, the Ministry of Commerce, the General Administration of Customs and the State Administration for Market Regulation issued Announcement No. 12 of 2020 on Further Strengthening the Quality Supervision of Export of Epidemic Prevention Materials.
Since the outbreak of the novel coronavirus, the company has rapidly organized its research and development capabilities to address the novel coronavirus. With the unremitting efforts of its R&D team, it has successfully developed a total anti-novel coronavirus detection reagent despite all difficulties. Its CoV-2 test kit is used at both individual clinics and in large hospitals. The colloidal gold method is suitable for independent clinics, and the immunoturbidimetry is suitable for mass population screening.
Novel Coronavirus Test Kit (Latex Enhanced Immunoturbidimetry) The immunoturbidimetry test kit is an automated methodology, which can be matched with the existing automation equipment of the hospital, and can be applied to the biochemical instruments of major brands in the world, such as Beckman, Roche, Abbott, etc., which reduces the risk of infection in the detection process of medical staff, and can realize the simultaneous testing of thousands of people. It is suitable for large-scale screening and detection of novel coronavirus from large first-class hospitals to primary medical institutions. The test speed can reach 2000 test/hour, repeatability <3%, batch difference <5%, which is suitable for a variety of different complex application scenarios such as suspected patient isolation area, confirmed patient isolation area, third-level hospital fever clinic, grass-roots hospital, community health clinic, etc. Advantages of the novel Coronavirus detection kit (latex enhanced immunoturbidimetry) : 1, with independent research and development of latex antigen coupling technology, liquid latex enhanced immune turbidimetry in vitro diagnosis technology; 2, the sample is blood, the source is flexible, reduce the risk of exposure to infection of medical staff, greatly relieve the pressure of clinical diagnosis and treatment; 3, the fastest liquid phase methodology in China, breaking through the restrictions on personnel, shorten the testing time, convenient operation; 4, a wide range of applications, can be widely used in medical institutions, with the hospital automatic biochemical analyzer supporting the use, for the majority of basic medical institutions is light economic burden, cost controllable, strong compatibility; 5. It can be combined with nucleic acid detection and CT examination for accurate diagnosis, to achieve rapid diagnosis of suspected patients and rapid screening of large numbers of close contacts to prevent the spread of the epidemic. Comprehensive interpretation and combined application of biochemical detection and other indications can help improve the detection rate of diseases, find out confirmed patients as much as possible, and be more conducive to the control of the epidemic.
Novel Coronavirus Test Kit (Colloidal Gold Method) Recently, there have been several new confirmed cases in China, reminding us to cherish the hard-won prevention and control results, and sounding the alarm for us on the other hand. According to the world real-time statistics show that as of 15:27 Beijing time on June 17, the total number of confirmed cases of COVID-19 in the world has exceeded 8.27 million, including 8,181,606 overseas cases, and the total number of deaths has exceeded 441,500, reaching 429,758 cases. In the face of the international epidemic, it takes on a corporate and social responsibility at present, just remembering its responsibilities at present, and contributing at present to the fight against COVID-19. The novel coronavirus detection reagents it has developed at present provide a health defense against viruses in the global fight against COVID-19. Its operations fulfill its solemn commitment to building a community with a shared future for mankind. |